New cell therapy shows promise for Covid-related respiratory distress
London, Feb 6 (IANS) A new type of cell therapy could improve the prognosis of those who are critically ill with acute respiratory distress syndrome (ARDS) resulting from severe Covid-19, according to promising trial results.
The findings, published in the journal Nature Communications, investigates the use of agenT-797, MiNK Therapeutic’s allogeneic, unmodified invariant natural killer T (iNKT) cell therapy.
The iNKT cell therapy has the effect of rescuing exhausted T cells and prompting an anti-inflammatory cytokine response, potentially activating anti-viral immunity to help these patients fight infection as well as to reduce severe, pathogenic inflammation of the lung.
The new research led by a team at the UK’s Anglia Ruskin University (ARU), found that agenT-797, which is also under investigation in cancer trials, could be manufactured rapidly, had a tolerable safety profile, and appeared to have a positive effect on mortality among critically unwell Covid-19 ARDS patients receiving intensive care.
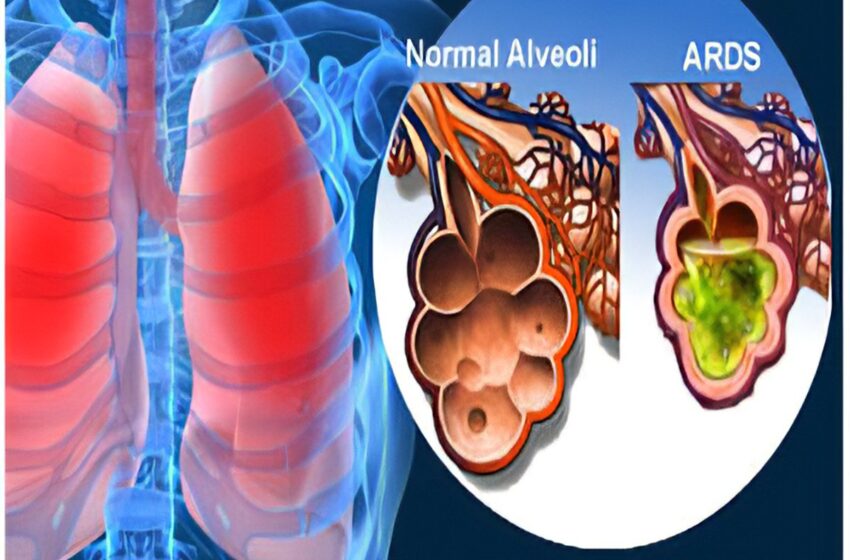
The exploratory trial included 20 mechanically ventilated patients with severe ARDS secondary to Covid.
Of the 20 patients in the trial, 14 survived (70 per cent) at 30 days (compared to a control group of 10 per cent), and there was an 80 per cent lower occurrence of bacterial pneumonia among those who received the highest dosage of agenT-797, compared to those who received fewer cells.
Twenty-one patients were treated overall (the main trial, plus one under compassionate use), which included five who were also receiving veno-venous extracorporeal membrane oxygenation (VV-ECMO), known as “the most aggressive salvage therapy” for critically-ill patients with ARDS.
In VV-ECMO, deoxygenated blood is pumped through a membrane lung and returned to the body via a cannula.
This trial is believed to be the first immune cell therapy of any type to be used in critically unwell patients undergoing VV-ECMO. Survival of the VV-ECMO cohort was 80 per cent after 30 and 90 days, and 60 per cent after 120 days.
This compares favorably to overall survival of 51 per cent for patients with Covid who were treated with just VV-ECMO at the same institution, during the same timeframe.
“During this small, exploratory study, we observed that MiNK’s iNKT cell treatment, which is also being advanced for people with cancer, triggered an anti-inflammatory response in ARDS patients,” Professor Justin Stebbing from the varsity said.
“Despite a poor prognosis, critically ill patients treated with this therapy showed favorable mortality rates and those treated at the highest dose also had reduced rates of pneumonia, underscoring the potential application of iNKT cells, and agenT-797 in particular, in treating viral diseases and infections more broadly,” he added.
